How AI is Transforming Drug Launch Strategies: Faster Research & Better Development

1 year ago
Launching a new drug is a complex process.
It involves years of research, clinical trials, and regulatory approvals.
But what if there was a way to make this faster and more efficient?
This is where AI-powered solutions like Cellbyte’s come into play.
In this blog, we’ll explore how AI is changing drug development.
You’ll see how it improves the process.
We’ll also show you how health-tech entrepreneurs and pharma companies in Canada can use these ideas to create their own innovative solutions.
The Problem with Drug Launch Strategy
The global pharmaceutical industry is a massive $1.5 trillion market, with hundreds of new drugs hitting the market each year.
But getting these new drugs to patients at the right price and time isn’t easy.
It requires complex go-to-market strategies, and specialized Pricing & Market Access (P&MA) teams are crucial to this process.
What’s the Challenge?
Figuring out the best launch strategy isn’t straightforward.
P&MA professionals have to sift through a mountain of data, including clinical, pricing, regulatory, and Health Technology Assessment (HTA) information.
But accessing and managing this data comes with several big hurdles:
- Resource Drain: A lot of time, money, and effort goes into collecting data. This often leaves only limited resources for developing a solid strategy.
- Data Reliability: Ensuring the accuracy of data can be tough. It often means dealing with lengthy documents in multiple languages.
- Detail Deficiency: Current databases might not have the detailed information needed to understand specific cases or markets.
Traditional Launch Process for New Drugs
Bringing a new drug to market is a long and detailed process. Here’s a simple breakdown of the steps involved:
- Discovery & Development: It all starts in the lab, where researchers work on developing a new drug.
- Preclinical Research: Before testing on humans, the drug undergoes laboratory and animal testing to check basic safety.
- Clinical Research: The drug is tested on people in three phases to ensure it’s both safe and effective. This stage can take several years to gather enough data.
- Review: Regulatory bodies like the FDA or EMA examine the data to decide whether the drug should be approved for public use.
- Marketing: Once approved, the drug is marketed to reach the right audiences.
- Post-Market Safety Monitoring: Even after a drug is on the market, regulatory bodies continue to monitor its safety.
Developing a new drug is costly, with expenses ranging from less than $1 billion to over $2 billion.
Companies must ensure their drug has significant potential to justify the investment.
Understanding these steps highlights the complexity of drug launches and the potential for AI to streamline and accelerate this process.

Cellbyte: A New AI Assistant for Drug Launches

Meet Cellbyte, an AI tool that’s changing the game for pharmaceutical companies.
It helps Pricing & Market Access (P&MA) teams analyze complex data quickly.
You can ask it questions in plain language, and it pulls up the answers in seconds.
Cellbyte makes it easy to sift through clinical, regulatory, and pricing data.
It even understands natural language queries and finds insights from industry documents quickly.
What used to take days now happens in seconds.
Right now, Cellbyte is focusing on Europe’s biggest markets and will soon expand to the U.S.
With Cellbyte, you can get the information you need in a flash, making drug launches smoother and faster.
How Cellbyte Works
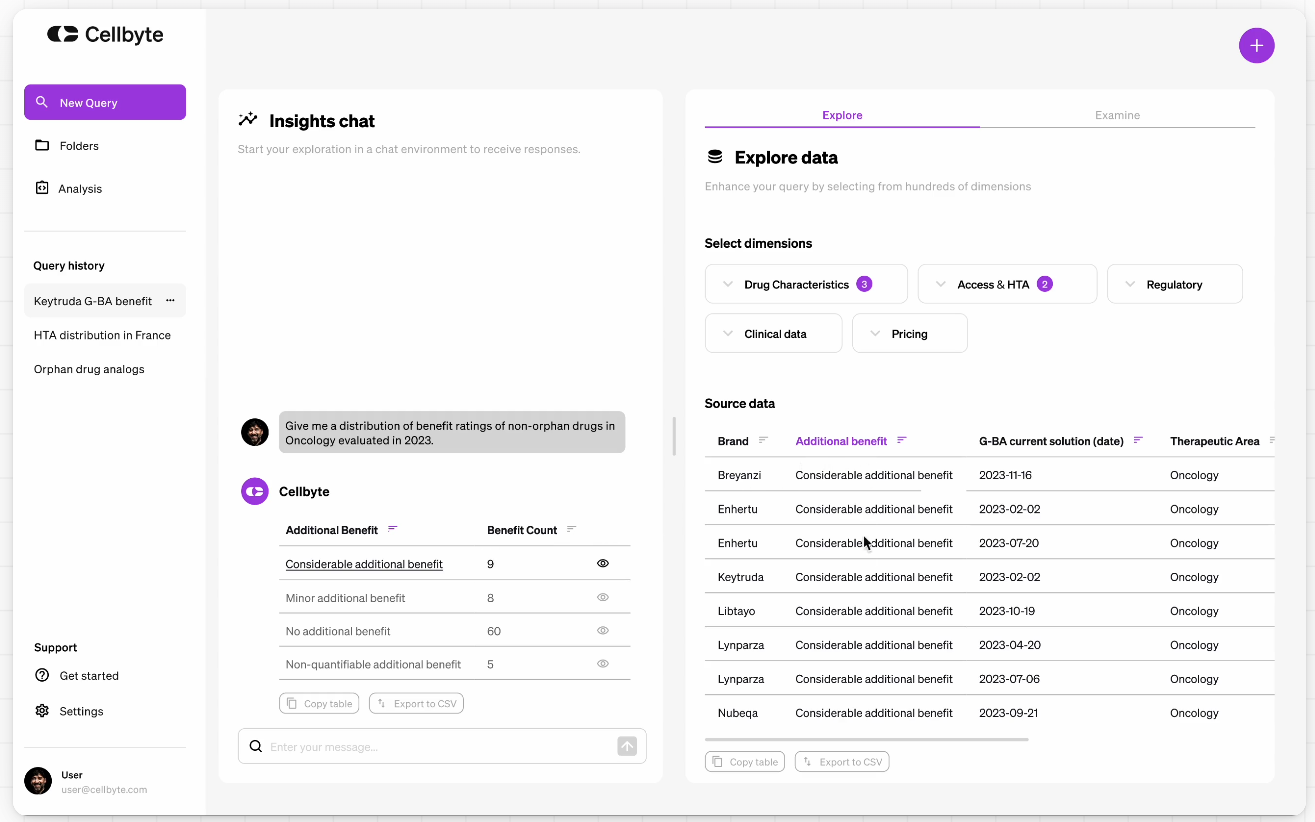
Cellbyte is designed to make data analysis easy and fast for Pricing & Market Access teams. Here’s how it works:
1. Effortless Data Analysis
You can dig into complex data using natural language. Just type your question, and Cellbyte provides answers in seconds.
2. Instant Insights
What used to take days now happens in seconds. Cellbyte scans through clinical, pricing, and regulatory data to deliver quick, accurate answers.
3. Self-Service Analytics
Need to run calculations or compare prices? Just type in your prompt. No more writing formulas or searching through spreadsheets.
4. Unmatched Search
Find specific information quickly. Ask for details on pricing or approval timelines, and get exactly what you need without sifting through piles of documents.
5. Easy Document Interaction
Extract insights from Pricing & Market Access documents with ease. Chat with the documents, get key details, and follow links to the original sources.
6. Comprehensive Data Integration
Cellbyte integrates data from Europe’s top HTA institutions, covering all the major markets.
How AI Can Boost Drug Launches and Commercial Success
AI is transforming how biopharma companies approach drug launches and market strategies. Here’s how:
1. Using Real-World Data (RWD)
AI helps analyze RWD to quickly adapt to market needs and justify costs. This ensures drugs meet market demands and stay competitive.
2. Predictive Pricing
AI tools analyze data to set better drug prices and improve profitability by predicting how prices will be scrutinized.
3. Omnichannel Marketing
AI predicts patient behavior and suggests targeted marketing strategies, helping companies connect effectively with stakeholders.
4. Market Segmentation
AI identifies key HCP and patient segments, refining strategies and tailoring approaches to different markets.
5. Scenario Planning
AI helps forecast market trends and plan strategies by analyzing potential future scenarios, and improving decision-making.

Adoption of AI Tools by Leading Biopharma Companies
Top pharmaceutical companies are already seeing the benefits of AI tools in their drug development and launch processes. Here’s how some industry giants are leveraging AI:
Pfizer is harnessing the power of IBM Watson, a sophisticated machine learning system.
This AI tool aids Pfizer in its quest for innovative immuno-oncology treatments, speeding up research and discovery.
Sanofi is partnering with UK start-up Exscientia, which specializes in AI technology.
Sanofi is using this AI to discover new treatments for metabolic illnesses, showcasing how AI can drive forward new drug development.

A Roche affiliate is employing an AI system from GNS Healthcare.
This technology helps Genentech in its global search for effective cancer therapies, demonstrating AI's role in enhancing research efficiency.
Want to Build an AI Tool for Pharma? Here’s How to Get Started
If you’re aiming to develop an AI tool for the pharmaceutical industry, here’s a roadmap to guide you:
1. Identify Key Challenges: Focus on solving real problems in drug launches, pricing, or market access. Understand the industry’s pain points, like handling complex data or optimizing launch strategies.
2. Leverage Real-World Data: Design your AI to analyze and interpret vast amounts of real-world data (RWD). This helps companies understand drug impact, pricing strategies, and market needs more effectively.
3. Integrate Advanced Analytics: Use predictive analytics to offer insights on pricing, market trends, and stakeholder behavior. This will help pharmaceutical companies make informed decisions and set competitive prices.
4. Enable Smart Market Segmentation: Build AI that can segment markets accurately. Use machine learning to identify different healthcare provider and patient segments, tailoring strategies to each.
5. Ensure Data Integration: Make sure your AI can handle data from various sources, including clinical trials, pricing databases, and regulatory documents. Seamless integration is key for comprehensive analysis.
6. Focus on User Experience: Design an intuitive interface that allows users to interact with the AI easily. The goal is to make complex data analysis as simple as possible.

